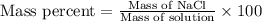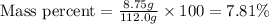Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

You know the right answer?
Asolution is prepared by dissolving 8.75 grams of sodium chloride in enough volume of water to produ...
Questions

Mathematics, 13.11.2020 19:40


Arts, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40





Mathematics, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

Chemistry, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

English, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

History, 13.11.2020 19:40


Chemistry, 13.11.2020 19:40