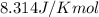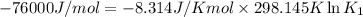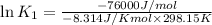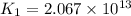Chemistry, 06.12.2019 04:31 emilybomar7466
Consider the reaction 4 hcl(g) + o2(g) =2 h2o(g) + 2 cl2(g) using the standard thermodynamic data in the tables linked above, calculate the equilibrium constant for this reaction at 298.15k.
delta g (kj/mol)
hcl=-95.3
o2=0
h2o=-228.6
cl2=0

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Consider the reaction 4 hcl(g) + o2(g) =2 h2o(g) + 2 cl2(g) using the standard thermodynamic data in...
Questions

Spanish, 06.08.2021 04:20








Mathematics, 06.08.2021 04:20


English, 06.08.2021 04:20

Mathematics, 06.08.2021 04:20


Computers and Technology, 06.08.2021 04:20

Spanish, 06.08.2021 04:20

English, 06.08.2021 04:20



Biology, 06.08.2021 04:20

Mathematics, 06.08.2021 04:20

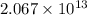 .
.![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f(product)]-\sum [n\times \Delta G^o_f(reactant)]](/tpl/images/0405/8552/b00b4.png)
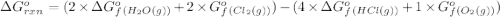
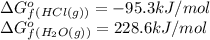
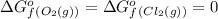 (pure element)
(pure element)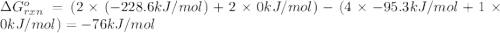
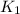 (at 25°C) for given value of Gibbs free energy, we use the relation:
(at 25°C) for given value of Gibbs free energy, we use the relation: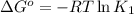
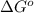 = Gibbs free energy = -76 kJ/mol = -76000 J/mol
= Gibbs free energy = -76 kJ/mol = -76000 J/mol