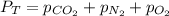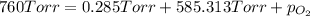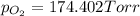Three of the primary components of air are
carbon dioxide, nitrogen, and oxygen. in a
sa...

Chemistry, 06.12.2019 05:31 jackchris2732
Three of the primary components of air are
carbon dioxide, nitrogen, and oxygen. in a
sample containing a mixture of only these
gases at exactly one atmosphere pressure, the
partial pressures of carbon dioxide and nitrogen are given as pco2 = 0.285 torr and
pn2 = 585.313 torr. what is the partial pressure of oxygen?
answer in units of torr.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Questions

Mathematics, 09.07.2019 03:30

Physics, 09.07.2019 03:30



Mathematics, 09.07.2019 03:30

Mathematics, 09.07.2019 03:30

Spanish, 09.07.2019 03:30

Mathematics, 09.07.2019 03:30

Chemistry, 09.07.2019 03:30

Mathematics, 09.07.2019 03:30

Mathematics, 09.07.2019 03:30



Mathematics, 09.07.2019 03:30

Mathematics, 09.07.2019 03:30

Biology, 09.07.2019 03:30




Biology, 09.07.2019 03:30

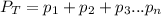
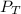 = Total pressure
= Total pressure 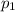 = partial pressure of gas-1
= partial pressure of gas-1 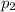 = partial pressure of gas-2
= partial pressure of gas-2 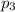 = partial pressure of gas-3
= partial pressure of gas-3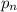 = partial pressure of nth gas in the mixture
= partial pressure of nth gas in the mixture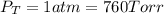
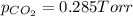
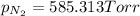
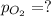
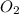 gas.
gas.