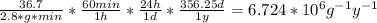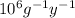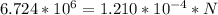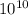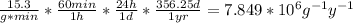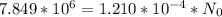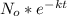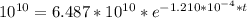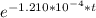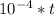Chemistry, 06.12.2019 05:31 ayoismeisjjjjuan
In a living organism, the decay of c-14 produces 15.3 disintegrations per minute per gram of carbon. the half-life of c-14 is 5730 years. a bone sample with 2.8 g of carbon has 36.7 disintegrations per minute. how old is the bone sample in years?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2
You know the right answer?
In a living organism, the decay of c-14 produces 15.3 disintegrations per minute per gram of carbon....
Questions


Physics, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00

Social Studies, 10.11.2020 14:00

History, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00


World Languages, 10.11.2020 14:00

Chemistry, 10.11.2020 14:00

English, 10.11.2020 14:00





Mathematics, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00




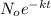
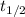
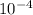 (1/year)
(1/year)