
Chemistry, 06.12.2019 05:31 smkw04p3ao0n
The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hcl(g) calculate the equilibrium concentration of hcl when 0.564 moles of nh4cl(s) are introduced into a 1.00 l vessel at 548 k.[hcl] = m

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hc...
Questions


Health, 06.11.2019 00:31

Arts, 06.11.2019 00:31

Health, 06.11.2019 00:31

Mathematics, 06.11.2019 00:31



Mathematics, 06.11.2019 00:31

Mathematics, 06.11.2019 00:31




Geography, 06.11.2019 00:31



Mathematics, 06.11.2019 00:31

Biology, 06.11.2019 00:31


Physics, 06.11.2019 00:31

Geography, 06.11.2019 00:31

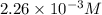
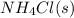 = 0.564 moles
= 0.564 moles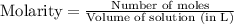
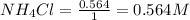
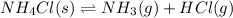
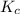 for above equation follows:
for above equation follows:![K_c=[NH_3][HCl]](/tpl/images/0405/9498/72be1.png)
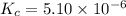
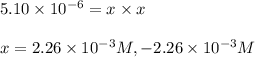
![[HCl]=2.26\times 10^{-3}M](/tpl/images/0405/9498/283fd.png)


