
Chemistry, 06.12.2019 05:31 DisneyyKayy
Calculate δh∘ in kilojoules for the reaction of acetylene (c2h2) (δh∘f=227.4kj/mol) with o2 to yield carbon dioxide (co2) (δh∘f=−393.5 kj/mol) and h2o(g) (δh∘f=−241.8kj/mol), a reaction which is supplied by the industrial gases industry for oxyacetylene gas welding and cutting due to the high temperature of the flame.

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Calculate δh∘ in kilojoules for the reaction of acetylene (c2h2) (δh∘f=227.4kj/mol) with o2 to yield...
Questions




History, 18.09.2019 22:50



Mathematics, 18.09.2019 22:50



History, 18.09.2019 22:50


English, 18.09.2019 22:50



Mathematics, 18.09.2019 22:50

Mathematics, 18.09.2019 22:50


Mathematics, 18.09.2019 22:50


Mathematics, 18.09.2019 22:50

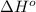 for the reaction is, -2512.4 kJ
for the reaction is, -2512.4 kJ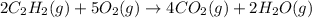
![\Delta H^o_{rxn}=[(3\times \Delta H^o_f_{(CO_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_2H_2(g))})+(5\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0405/9362/62f19.png)
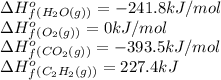
![\Delta H^o_{rxn}=[(4\times (-393.5))+(2\times (-241.8))]-[(2\times (227.4)+(5\times (0))]\\\\\Delta H^o_{rxn}=-2512.4kJ](/tpl/images/0405/9362/361f8.png)


