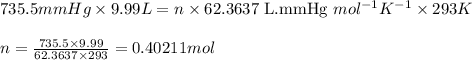Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)arrow. gif2kcl(s) + 3o2(g)the product gas, o2, is collected over water at a temperature of 20 °c and a pressure of 748 mm hg. if the wet o2 gas formed occupies a volume of 9.49 l, the number of moles of kclo3 reacted was ? mol. the vapor pressure of water is 17.5 mm hg at 20 °c. oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)arrow. gif2kcl(s) + 3o2(g)the product gas, o2, is collected over water at a temperature of 20 °c and a pressure of 753 mm hg. if the wet o2 gas formed occupies a volume of 9.99 l, the number of grams of o2 formed is ? g. the vapor pressure of water is17.5 mm hg at 20 °c.

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3...
Questions

Mathematics, 28.08.2020 22:01

Social Studies, 28.08.2020 22:01


Chemistry, 28.08.2020 22:01




Chemistry, 28.08.2020 22:01



Mathematics, 28.08.2020 22:01





Physics, 28.08.2020 22:01

Mathematics, 28.08.2020 22:01

Computers and Technology, 28.08.2020 22:01

Mathematics, 28.08.2020 22:01

Mathematics, 28.08.2020 22:01

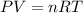
![20^oC=[20+273]K=293K](/tpl/images/0406/0694/3b5d4.png)
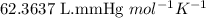
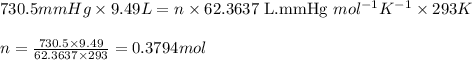
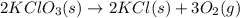
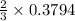 moles of potassium chlorate undergoes reaction.
moles of potassium chlorate undergoes reaction.