
Aresearcher used hplc to examine a bicomponent mixture containing 1.22 mg/l of compound a and 1.31 mg/l of compound b, which was added as an internal standard. this mixture produced peak areas for compounds a and b of 10919 and 5379 , respectively. using the above information, determine the response factor (f).
after establishing f, the researcher prepared a solution by combining 8.18 mg of b with 10.00 ml of an unknown solution containing only a and then diluted it to a final volume of 50.00 ml. the sample was examined using hplc and peak areas of 6065 and 9111 were observed for a and b, respectively.
determine the concentration of a (mg/ml) in the unknown solution.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
Aresearcher used hplc to examine a bicomponent mixture containing 1.22 mg/l of compound a and 1.31 m...
Questions


History, 29.01.2020 00:05


Mathematics, 29.01.2020 00:05


Chemistry, 29.01.2020 00:05



Mathematics, 29.01.2020 00:05


Mathematics, 29.01.2020 00:05

Arts, 29.01.2020 00:05

Mathematics, 29.01.2020 00:05



Mathematics, 29.01.2020 00:06




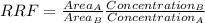
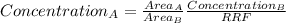
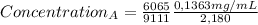
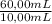 = 02497 mg/mL
= 02497 mg/mL

