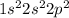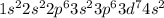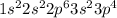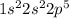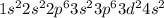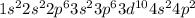Chemistry, 06.12.2019 06:31 anicholson41
According to hund's rule of maximum spin multiplicity, how many singly-occupied orbitals are there in the valence shells of the following elements in their ground states? enter your answer as the sum of all the orbitals (for example 15).
a) carbon
b) cobalt
c) sulfur
d) fluorine
e) titanium
f) germanium

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
According to hund's rule of maximum spin multiplicity, how many singly-occupied orbitals are there i...
Questions

Chemistry, 03.12.2020 14:00



Biology, 03.12.2020 14:00



Social Studies, 03.12.2020 14:00

English, 03.12.2020 14:00


Mathematics, 03.12.2020 14:00




Biology, 03.12.2020 14:00



Medicine, 03.12.2020 14:00


Business, 03.12.2020 14:00

English, 03.12.2020 14:00