
In yeast, ethanol is produced from glucose under anaerobic conditions. a cell‑free yeast extract is placed in a solution that contains 3.50 × 10 2 mmol glucose, 0.35 mmol adp , 0.35 mmol p i , 0.70 mmol atp , 0.20 mmol nad + , and 0.20 mmol nadh . it is kept under anaerobic conditions. what is the maximum amount of ethanol (in millimoles) that could theoretically be produced under these conditions?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
In yeast, ethanol is produced from glucose under anaerobic conditions. a cell‑free yeast extract is...
Questions





Mathematics, 19.05.2021 19:30

Computers and Technology, 19.05.2021 19:30

English, 19.05.2021 19:30

Social Studies, 19.05.2021 19:30





Mathematics, 19.05.2021 19:30

Mathematics, 19.05.2021 19:30

Mathematics, 19.05.2021 19:30


Mathematics, 19.05.2021 19:30


Mathematics, 19.05.2021 19:30

Mathematics, 19.05.2021 19:30

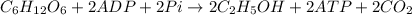
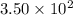 milli mole
milli mole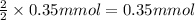 of ethanol
of ethanol

