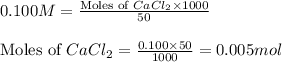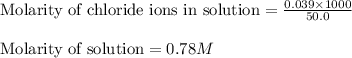Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
Asolution is prepared by adding 1.70 g of solid nacl to 50.0 ml of 0.100 m cacl2. what is the molari...
Questions


Physics, 22.01.2021 14:00

Biology, 22.01.2021 14:00


Spanish, 22.01.2021 14:00



English, 22.01.2021 14:00




Mathematics, 22.01.2021 14:00



Mathematics, 22.01.2021 14:00



Mathematics, 22.01.2021 14:00

History, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00

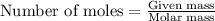
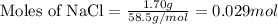
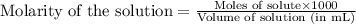 .....(1)
.....(1)