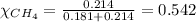Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of helium and 3.43 grams of methane. what is the partial pressure of each gas in the mixture?
phe = mm hg
pch4 = mm hg
2.) a mixture of nitrogen and carbon dioxide gases contains nitrogen at a partial pressure of 363 mm hg and carbon dioxide at a partial pressure of 564 mm hg. what is the mole fraction of each gas in the mixture?
xn2 =
xco2 =

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of heli...
Questions




Mathematics, 03.12.2020 03:40

Mathematics, 03.12.2020 03:40

Mathematics, 03.12.2020 03:40

Arts, 03.12.2020 03:40

Computers and Technology, 03.12.2020 03:40


World Languages, 03.12.2020 03:40

Mathematics, 03.12.2020 03:40

Advanced Placement (AP), 03.12.2020 03:40


Biology, 03.12.2020 03:40

Mathematics, 03.12.2020 03:40

History, 03.12.2020 03:40

Mathematics, 03.12.2020 03:40


Social Studies, 03.12.2020 03:40

 .....(1)
.....(1)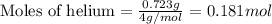

 .......(2)
.......(2) ......(3)
......(3)