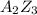Chemistry, 06.12.2019 21:31 bethzabezarahi
Suppose an ionic solid with a cubic unit cell contains a (cations) and z (anions). the a ions are in the body center position and at each corner, and the z ions are on each face. based on this structure the empirical formula of the compound would be az

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


You know the right answer?
Suppose an ionic solid with a cubic unit cell contains a (cations) and z (anions). the a ions are in...
Questions

Physics, 14.06.2021 14:00


Chemistry, 14.06.2021 14:00

Chemistry, 14.06.2021 14:00

Mathematics, 14.06.2021 14:00



English, 14.06.2021 14:00

English, 14.06.2021 14:00

Physics, 14.06.2021 14:00

Chemistry, 14.06.2021 14:00

Mathematics, 14.06.2021 14:00

History, 14.06.2021 14:00

Arts, 14.06.2021 14:00

Physics, 14.06.2021 14:00

Mathematics, 14.06.2021 14:00

Mathematics, 14.06.2021 14:00



Advanced Placement (AP), 14.06.2021 14:00