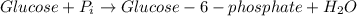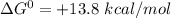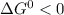Chemistry, 06.12.2019 23:31 deidaralove90
In the first step of glycolysis, the given two reactions are coupled. reaction 1: glucose + p i ⟶ glucose - 6 - phosphate + h 2 o δ g = + 13.8 k j / mol reaction 2: atp + h 2 o ⟶ adp + p i δ g = − 30.5 k j / mol answer the four questions about the first step of glycolysis. is reaction 1 spontaneous or nonspontaneous?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
In the first step of glycolysis, the given two reactions are coupled. reaction 1: glucose + p i ⟶ g...
Questions


English, 07.11.2020 14:10

Mathematics, 07.11.2020 14:10



Physics, 07.11.2020 14:20

Biology, 07.11.2020 14:20

Social Studies, 07.11.2020 14:20

Chemistry, 07.11.2020 14:20


Mathematics, 07.11.2020 14:20


Computers and Technology, 07.11.2020 14:20

Computers and Technology, 07.11.2020 14:20

Mathematics, 07.11.2020 14:20


English, 07.11.2020 14:20


Mathematics, 07.11.2020 14:20

Chemistry, 07.11.2020 14:20