
Chemistry, 06.12.2019 23:31 sandyykn192
Iven the following chemical equations: i. c+ 2h_2 2 → ch_4 4 (0.22 moles of h_2 2 )ii. 6co_2 2 + 6h_2 2 o → c_6 6 h_{12} 12 o_6 6 (2.25 g of co_2 2 )iii. ch_4 4 + 2o_2 2 → co_2 2 + 2h_2 2 o (1.25 l of ch_4 4 with density = 0.656 g/l)which equation will have the highest number of carbon atoms produced based on the information given next to each chemical equation

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 10:30
Chemical bonds result from the interaction of the from two or more atoms. a. protons b. electrons c. neutrons d. nuclei
Answers: 2

Chemistry, 23.06.2019 13:20
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. this reaction is now the first step taken to make most of the world's fertilizer. suppose a chemical engineer studying a new catalyst for the haber reaction finds that 671 liters per second of dinitrogen are consumed when the reaction is run at 271c and 0.99atm. calculate the rate at which ammonia is being produced. give your answer in kilograms per second. round your answer to significant digits.
Answers: 3
You know the right answer?
Iven the following chemical equations: i. c+ 2h_2 2 → ch_4 4 (0.22 moles of h_2 2 )ii. 6co_2 2 + 6h_...
Questions

Mathematics, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30

Health, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30


Chemistry, 23.02.2021 19:30



English, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30



Mathematics, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30


Social Studies, 23.02.2021 19:30



Computers and Technology, 23.02.2021 19:30

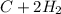 ⇒
⇒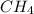 (0.22 moles of
(0.22 moles of 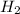 )
)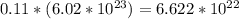 atoms
atoms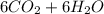 ⇒
⇒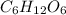
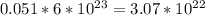 atoms.
atoms.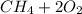 ⇒
⇒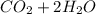
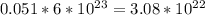 atoms.
atoms.

