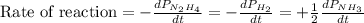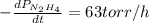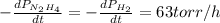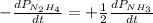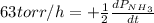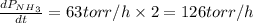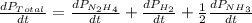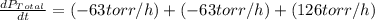Chemistry, 07.12.2019 00:31 kaylarenee05080
The rate of decrease in n2h4 partial pressure in a closed reaction vessel form the reaction: n2h4 (g) + h2 (g) \rightarrow 2 nh3 (g) is63 torr/h. what are the rates of change of nh3 partial pressure and total pressure in the vessel.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
The rate of decrease in n2h4 partial pressure in a closed reaction vessel form the reaction: n2h4 (...
Questions


Health, 31.10.2020 06:00

Mathematics, 31.10.2020 06:00







Mathematics, 31.10.2020 06:10


Chemistry, 31.10.2020 06:10


Mathematics, 31.10.2020 06:10


Health, 31.10.2020 06:10


Mathematics, 31.10.2020 06:10

History, 31.10.2020 06:10

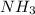 partial pressure is 126 torr/h.
partial pressure is 126 torr/h.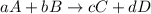
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0407/2122/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0407/2122/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0407/2122/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0407/2122/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0407/2122/d4b94.png)
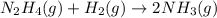
![\text{Rate of disappearance of }N_2H_4=-\frac{d[N_2H_4]}{dt}](/tpl/images/0407/2122/4b6d3.png)
![\text{Rate of disappearance of }H_2=-\frac{d[H_2]}{dt}](/tpl/images/0407/2122/53b46.png)
![\text{Rate of formation of }NH_3=+\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0407/2122/f55ec.png)