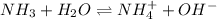Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3


Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
You know the right answer?
The following equation shows the equilibrium in an aqueous solution of ammonia: nh3(aq)+h2o(l)⇌nh4+...
Questions


Geography, 04.07.2019 09:50

Mathematics, 04.07.2019 09:50

Physics, 04.07.2019 09:50


Social Studies, 04.07.2019 09:50


Mathematics, 04.07.2019 09:50


Chemistry, 04.07.2019 09:50

Mathematics, 04.07.2019 09:50

History, 04.07.2019 09:50

Mathematics, 04.07.2019 09:50




History, 04.07.2019 09:50

Mathematics, 04.07.2019 09:50

English, 04.07.2019 09:50

Health, 04.07.2019 09:50

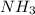 is a base and
is a base and 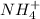 is its conjugate acid and thus conjugate acid-base pair.
is its conjugate acid and thus conjugate acid-base pair.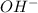 is a base and
is a base and 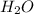 is its conjugate acid and thus conjugate acid-base pair.
is its conjugate acid and thus conjugate acid-base pair.