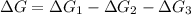Consider these hypothetical chemicalreactions:
{\rm a \rightleftharpoons b}, \quad\delta g =...

Chemistry, 07.12.2019 02:31 queenkimm26
Consider these hypothetical chemicalreactions:
{\rm a \rightleftharpoons b}, \quad\delta g = 13.2 kj/mol
{\rm b \rightleftharpoons c}, \quad\delta g = -28.9 kj/mol
{\rm c \rightleftharpoons d},\quad \delta g = 5.80 kj/mol
what is the free energy, delta g, for the overall reaction, \rm a \rightleftharpoons d ?
express your answer numerically inkilojoules per mole.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Questions


History, 02.02.2020 18:02


Mathematics, 02.02.2020 18:02

Mathematics, 02.02.2020 18:02

Mathematics, 02.02.2020 18:02

Chemistry, 02.02.2020 18:02


Mathematics, 02.02.2020 18:02


History, 02.02.2020 18:02

English, 02.02.2020 18:02



Mathematics, 02.02.2020 18:02

Social Studies, 02.02.2020 18:02


Biology, 02.02.2020 18:02

Biology, 02.02.2020 18:02

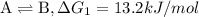 ...[1]
...[1]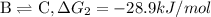 ...[2]
...[2]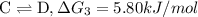 ...[3]
...[3]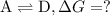 ...[4]
...[4]