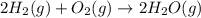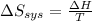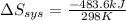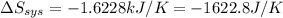Consider the following reaction at 298 k:
2h2(g) + o2(g) --> 2h2o(g) deltah= -483.6...

Chemistry, 07.12.2019 02:31 helloitschump0vfdz
Consider the following reaction at 298 k:
2h2(g) + o2(g) --> 2h2o(g) deltah= -483.6 kj
calculate the following quantities.
delta s sys=
delta s surr=
delta s univ=

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

You know the right answer?
Questions



English, 09.12.2019 20:31

History, 09.12.2019 20:31

Biology, 09.12.2019 20:31

Spanish, 09.12.2019 20:31


Chemistry, 09.12.2019 20:31

Physics, 09.12.2019 20:31



Chemistry, 09.12.2019 21:31






Mathematics, 09.12.2019 21:31

History, 09.12.2019 21:31

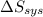 = -1622.8 J/K
= -1622.8 J/K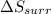 = -94.6 J/K
= -94.6 J/K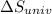 = 0 J/K
= 0 J/K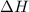 = -483.6 kJ
= -483.6 kJ