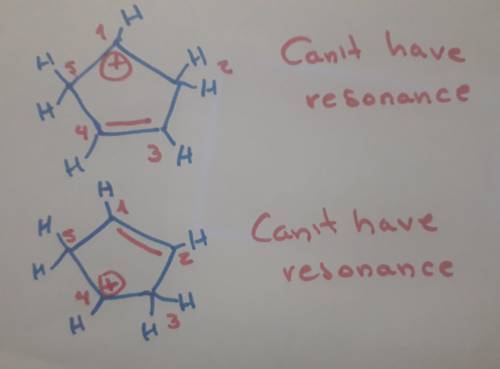
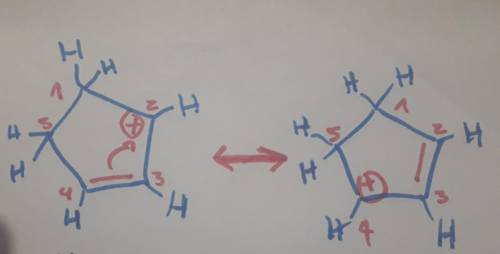
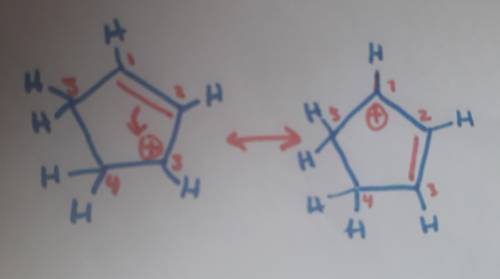
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation. likewise, carbons 2 and 3 are equivalent. write the structure of the carbocation formed by protonation of c−2 or c−3 to verify that it is not allylic and therefore not as stable as the one formed by protonation of c−1 or c−4.

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2


Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation....
Questions

Computers and Technology, 04.10.2020 04:01







English, 04.10.2020 04:01

Computers and Technology, 04.10.2020 04:01




Physics, 04.10.2020 04:01

Social Studies, 04.10.2020 04:01

English, 04.10.2020 04:01

Business, 04.10.2020 04:01

Computers and Technology, 04.10.2020 04:01


English, 04.10.2020 04:01