Chemistry, 07.12.2019 03:31 rbeltran24
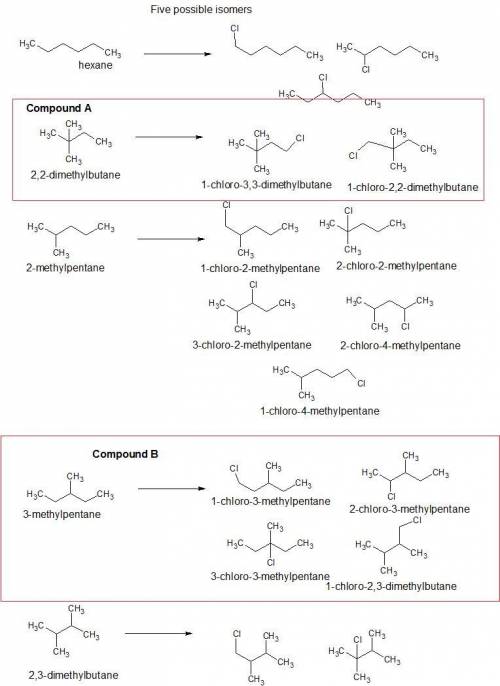
Both compounds a and b have molecular formula c6h14. monochlorination of compound a results in the formation of two constitutional isomers. monochlorination of compound b results in the formation of four constitutional isomers. identify compounds a and b, and show the products of each monochlorination.
1) draw compound a and the products of its monochlorination.
2)draw compound b and the products of its monochlorination.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
Both compounds a and b have molecular formula c6h14. monochlorination of compound a results in the f...
Questions

Mathematics, 31.03.2020 03:49