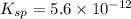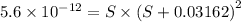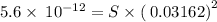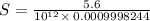Chemistry, 07.12.2019 05:31 NeverEndingCycle
What is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is 5.6 × 10-12. what is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is 5.6 × 10-12. 1.1 × 10-4 m 5.6 × 10-9 m 2.4 × 10-6 m 1.8 × 10-10 m

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
What is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is...
Questions


History, 05.02.2021 01:00

Arts, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00

Social Studies, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

History, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00




Biology, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00

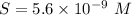
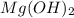 will form its respective ions in the solution as:
will form its respective ions in the solution as:
![K_{sp}=[Mg^{2+}][OH^-]^2](/tpl/images/0407/6768/48330.png)
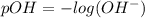
![[OH^-]=10^{(-1.5)}=0.03162](/tpl/images/0407/6768/a8c86.png)