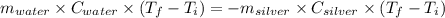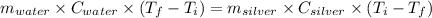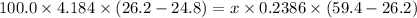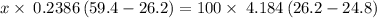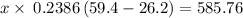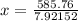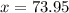Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
Asilver block, initially at 59.4 °c, is submerged into 100.0 g of water at 24.8 °c, in an insulated...
Questions



History, 16.11.2020 04:00

History, 16.11.2020 04:00

Mathematics, 16.11.2020 04:00

History, 16.11.2020 04:00


Mathematics, 16.11.2020 04:00



Mathematics, 16.11.2020 04:00




Biology, 16.11.2020 04:00


Computers and Technology, 16.11.2020 04:00

Mathematics, 16.11.2020 04:00

Biology, 16.11.2020 04:00