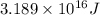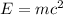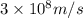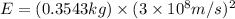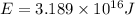Chemistry, 08.12.2019 00:31 llamasking
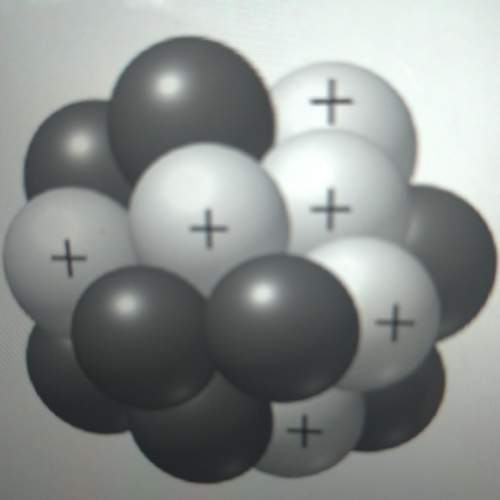
50 points! the isotope shown has a mass of 14.003241 amu. calculate how much energy is released from the binding of 2.530x10 moles of this isotope. all particles in the nucleus are shown.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
50 points! the isotope shown has a mass of 14.003241 amu. calculate how much energy is released fro...
Questions


Mathematics, 14.07.2019 00:50


Mathematics, 14.07.2019 00:50


English, 14.07.2019 00:50


Mathematics, 14.07.2019 00:50



History, 14.07.2019 00:50

World Languages, 14.07.2019 00:50

Social Studies, 14.07.2019 00:50

Mathematics, 14.07.2019 00:50


English, 14.07.2019 00:50

Mathematics, 14.07.2019 00:50

Chemistry, 14.07.2019 00:50