6.00x10-3 mol of hbr arec
+
view? assignmentproblemid=127486083& offset=next
7 of...


Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Questions

English, 07.04.2020 22:39




Mathematics, 07.04.2020 22:39

History, 07.04.2020 22:39

Mathematics, 07.04.2020 22:39

Mathematics, 07.04.2020 22:39

Mathematics, 07.04.2020 22:39



Chemistry, 07.04.2020 22:39








Mathematics, 07.04.2020 22:39

![[OH^{-}]](/tpl/images/0408/8029/e46dd.png) is
is 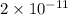
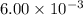
![[HBr]= \frac{6.00\times 10^{-3}}{12}M](/tpl/images/0408/8029/d8bf2.png)
![[H^{+}]= \frac{6.00\times 10^{-3}}{12}M](/tpl/images/0408/8029/9f0c2.png)
![[H^{+}][OH^{-}]= 10^{-14}](/tpl/images/0408/8029/8cf7b.png)
![[OH^{-}]= \frac{1.0\times 10^{-14}}{[\frac{6 \times10^{-3}}{12}]}= \frac{12 \times 10^{-14}}{6 \times10^{-3}}=2\times10^{-11}](/tpl/images/0408/8029/ca8b8.png)


