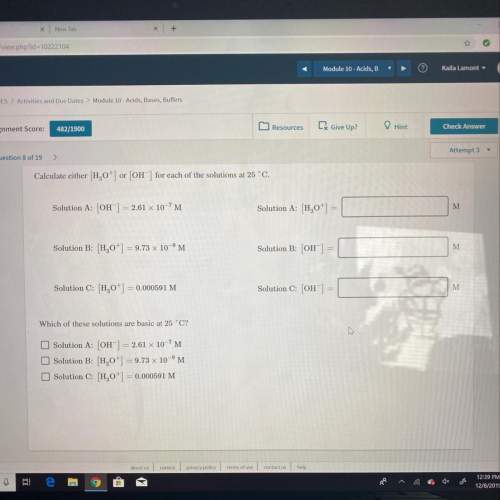
Calculate either h,0") or (oh") for each of the solutions at 25°c.
solution a: (oh) = 2.61 x...

Chemistry, 09.12.2019 01:31 hannahsparks7073
Calculate either h,0") or (oh") for each of the solutions at 25°c.
solution a: (oh) = 2.61 x 10-7m
solution a: 1,0+1 =
solution b: (,0) = 9.73 x 10-'m
solution b: oh
solution c: h,0+1 = 0.000591 m
solution c: [oh] = 0
which of these solutions are basic at 25 °c?
solution a: (oh) = 2.61 x 10-7m
solution b: h,0+) = 9.73 x 10-'m
solution c: h0+] = 0.000591 m

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Questions

History, 05.05.2020 05:12



Mathematics, 05.05.2020 05:12



Mathematics, 05.05.2020 05:12

Advanced Placement (AP), 05.05.2020 05:12

Biology, 05.05.2020 05:12

Physics, 05.05.2020 05:12

Chemistry, 05.05.2020 05:12

Computers and Technology, 05.05.2020 05:12

Computers and Technology, 05.05.2020 05:12





History, 05.05.2020 05:12

Mathematics, 05.05.2020 05:12



