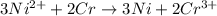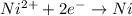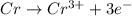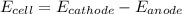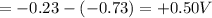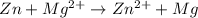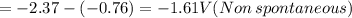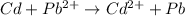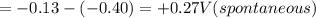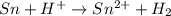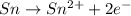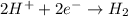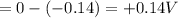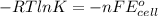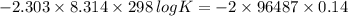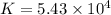Chemistry, 09.12.2019 17:31 jaydenbrock
You may want to reference (pages 906 - 909) section 19.5 while completing this problem. standard reduction half-cell potentials at 25∘c half-reaction e∘ (v) half-reaction e∘ (or) au3+(aq)+3e−→au(s) 1.50 fe2+(aq)+2e−→fe(s) −0.45 ag+(aq)+e−→ag(s) 0.80 cr3+(aq)+e−→cr2+(aq) −0.50 fe3+(aq)+3e−→fe2+(aq) 0.77 cr3+(aq)+3e−→cr(s) −0.73 cu+(aq)+e−→cu(s) 0.52 zn2+(aq)+2e−→zn(s) −0.76 cu2+(aq)+2e−→cu(s) 0.34 mn2+(aq)+2e−→mn(s) −1.18 2h+(aq)+2e−→h2(g) 0.00 al3+(aq)+3e−→al(s) −1.66 fe3+(aq)+3e−→fe(s) −0.036 mg2+(aq)+2e−→mg(s) −2.37 pb2+(aq)+2e−→pb(s) −0.13 na+(aq)+e−→na(s) −2.71 sn2+(aq)+2e−→sn(s) −0.14 ca2+(aq)+2e−→ca(s) −2.76 ni2+(aq)+2e−→ni(s) −0.23 ba2+(aq)+2e−→ba(s) −2.90 co2+(aq)+2e−→co(s) −0.28 k+(aq)+e−→k(s) −2.92 cd2+(aq)+2e−→cd(s) −0.40 li+(aq)+e−→li(s) −3.04 part a use the tabulated electrode potentials to calculate k for the oxidation of tin by h+ (at 25 ∘c): sn(s)+2h+(aq)→sn2+(aq)+h2(g) express your answer using two significant figures. kk = previous answer request answer incorrect; try again; 5 attempts remaining provide feedback incorrect. incorrect; try again; 5 attempts remaining. no additional feedback.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
You may want to reference (pages 906 - 909) section 19.5 while completing this problem. standard red...
Questions


Mathematics, 26.01.2020 18:31

English, 26.01.2020 18:31

Computers and Technology, 26.01.2020 18:31

Mathematics, 26.01.2020 18:31

Mathematics, 26.01.2020 18:31




Chemistry, 26.01.2020 18:31




Mathematics, 26.01.2020 18:31

Social Studies, 26.01.2020 18:31


English, 26.01.2020 18:31

Social Studies, 26.01.2020 18:31


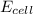 of the reaction is +0.50V
of the reaction is +0.50V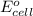 of the reaction is -1.61 V.
of the reaction is -1.61 V.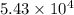 .
.