
Chemistry, 09.12.2019 17:31 bartekzelazek5083
X+ 2y > xy2in order to determine the order of the reaction represented above, the initial rate of formation of xy2 is measured using different initial values of [x] and [y]. the results of the experiment are shown in the table below. trial [x] [y] rate of formation [xy2]1 .5 m .5 m 8.0 x 10^-3 m/s2 1.0 m .5 m 3.2 x 10^-2 m/s3 1.0 m 1.0 m 6.4 x 10^-2 m/sin trial 2 which of the reactants would be consumed more rapidly and why? x, because the reaction is second order with respect to x. y, because the reaction is second order with respect to y. x, because it has a higher molar concentration. y, because the rate of disappearance will be double that of x.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
X+ 2y > xy2in order to determine the order of the reaction represented above, the initial rate o...
Questions



Mathematics, 24.11.2020 18:00




English, 24.11.2020 18:00








Chemistry, 24.11.2020 18:00


Mathematics, 24.11.2020 18:00

Mathematics, 24.11.2020 18:00


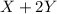 ⇒
⇒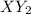
![\left[\begin{array}{ccc}[X]&[Y]&rate\\1.5M&0.5M&8*10^{-3}Ms^{-1}\\21M&0.5M&3.2*10^{-2}Ms^{-1}\\31M&1M&6.4*10^{-2}Ms{-1}\end{array}\right]](/tpl/images/0410/1221/efcd6.png)
 is ,
is ,![r \alpha [X]^a[Y]^b](/tpl/images/0410/1221/1f54d.png)
 and
and 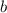
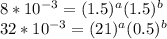
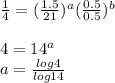
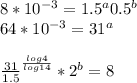 a=0.5253b=0.7048
a=0.5253b=0.7048

