
Chemistry, 09.12.2019 17:31 makayla4141
X2 + y2 > x2y2 rate = k[x2]a reaction and its experimentally determined rate law are represented above. a chemist proposes two different possible mechanisms for the reaction, which are given below. mechanism 1 mechanism 2x2 > 2x (slow) x2 > 2x (slow)x + y2 > xy2 (fast) x + y2 > xy + y (fast)x + xy2 > x2y2 (fast) x + xy > x2y (fast)x2y + y > x2y2 (fast)based on the information above, which of the following is true? both mechanism 1 and 2 are consistent with the rate law. only mechanism 2 is consistent with the rate law. neither mechanism 1 nor mechanism 2 is consistent with the rate law. only mechanism 1 is consistent with the rate law.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1



Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
X2 + y2 > x2y2 rate = k[x2]a reaction and its experimentally determined rate law are represented...
Questions

Mathematics, 15.12.2020 03:00

History, 15.12.2020 03:00

English, 15.12.2020 03:00

History, 15.12.2020 03:00



Social Studies, 15.12.2020 03:00


World Languages, 15.12.2020 03:00



Mathematics, 15.12.2020 03:00



Mathematics, 15.12.2020 03:00

Mathematics, 15.12.2020 03:00


Mathematics, 15.12.2020 03:00

Mathematics, 15.12.2020 03:00

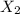 ⇒
⇒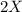 is the slowest step or RATE DETERMINING STEP .
is the slowest step or RATE DETERMINING STEP .![[X_2]](/tpl/images/0410/1202/df4e1.png) .
.

