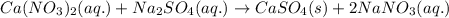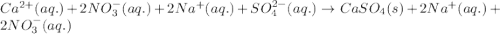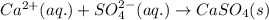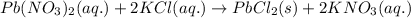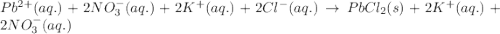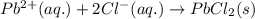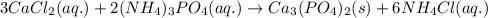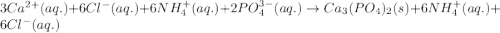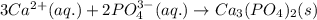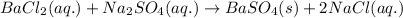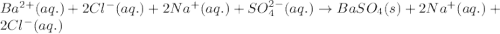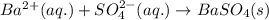Chemistry, 09.12.2019 17:31 jbehrens6538
Write the net ionic equation to show the formation of a solid (insoluble salt) when the following solutions are mixed. write noreaction if there is no precipitate. express your answer as a chemical equation. identify all of the phases in your answer. enter noreaction if no precipitate is formed.1. ca(no3)2(aq) + na2so4(aq)2. kcl(aq) + pb(no3)2(aq)3. cacl2(aq) + (nh4)3(po4)(aq)4. na2so4(aq) + bacl2(aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1
You know the right answer?
Write the net ionic equation to show the formation of a solid (insoluble salt) when the following so...
Questions

History, 02.12.2020 23:30


Business, 02.12.2020 23:30



Mathematics, 02.12.2020 23:30


Mathematics, 02.12.2020 23:30

Mathematics, 02.12.2020 23:30






Mathematics, 02.12.2020 23:30


Health, 02.12.2020 23:30



Law, 02.12.2020 23:30