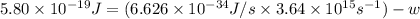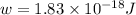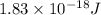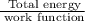Chemistry, 09.12.2019 18:31 gajdmaciej9502
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a kinetic energy of 5.80× 10–19 j. what is the maximum number of electrons that could be ejected from this metal by a burst of light (at some other frequency) with a total energy of 8.66× 10–7 j?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1


Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a ki...
Questions


Advanced Placement (AP), 12.01.2020 13:31

History, 12.01.2020 13:31

Mathematics, 12.01.2020 13:31



Mathematics, 12.01.2020 13:31

English, 12.01.2020 13:31


Chemistry, 12.01.2020 13:31


English, 12.01.2020 13:31

Mathematics, 12.01.2020 13:31




Mathematics, 12.01.2020 13:31

History, 12.01.2020 13:31

Mathematics, 12.01.2020 13:31

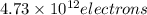
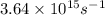
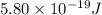
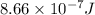
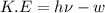
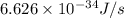
 = frequency
= frequency