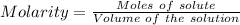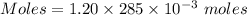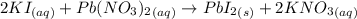You mix 285.0 ml of 1.20 m lead(ii) nitrate with 300.0 ml of 1.60 m potassium iodide. the lead(ii) iodide is insoluble. which of the following is false?
select one:
a. the final concentration of pb2+ ions is 0.174 m.
b. you form 111 g of lead(ii) iodide.
c. the final concentration of k+ is 0.821 m.
d. the final concentration of no3– is 0.821 m.
e. all are true.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
You mix 285.0 ml of 1.20 m lead(ii) nitrate with 300.0 ml of 1.60 m potassium iodide. the lead(ii) i...
Questions


Computers and Technology, 31.10.2020 15:50


Mathematics, 31.10.2020 15:50

Mathematics, 31.10.2020 15:50

Computers and Technology, 31.10.2020 15:50

English, 31.10.2020 15:50


Social Studies, 31.10.2020 15:50






Spanish, 31.10.2020 15:50

Mathematics, 31.10.2020 15:50


English, 31.10.2020 16:00

Mathematics, 31.10.2020 16:00

Geography, 31.10.2020 16:00