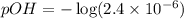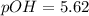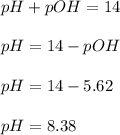Chemistry, 09.12.2019 18:31 skyrileycasting
What is the ph of a sodium formate solution prepared by adding 0.680 grams of sodium formate to 100.0 ml of water at 25.0°c? the ka at 25.0 °c for formic acid is 1.8 x 10-4. enter your answer with two decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1


Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
What is the ph of a sodium formate solution prepared by adding 0.680 grams of sodium formate to 100....
Questions


History, 01.04.2020 07:33



Medicine, 01.04.2020 07:33

Mathematics, 01.04.2020 07:33


Mathematics, 01.04.2020 07:33

Mathematics, 01.04.2020 07:33

Mathematics, 01.04.2020 07:33


Mathematics, 01.04.2020 07:33


Mathematics, 01.04.2020 07:33

Mathematics, 01.04.2020 07:34



English, 01.04.2020 07:34


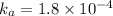
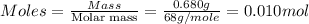
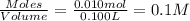
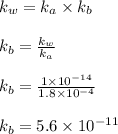
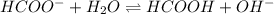
![k_b=\frac{[HCOOH][OH^-]}{[HCOO^-]}](/tpl/images/0410/1996/3e107.png)
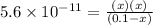
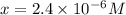
![[OH^-]=x=2.4\times 10^{-6}M](/tpl/images/0410/1996/7643c.png)
![pOH=-\log [OH^-]](/tpl/images/0410/1996/1fac1.png)