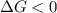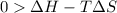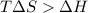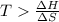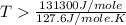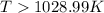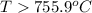Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3


Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
For the reaction c(s)+h2o(g)→co2(g)+h2(g) δh∘=131.3kj/mol and δs∘=127.6j/k⋅mol at 298k. at temperatu...
Questions






Mathematics, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01


Chemistry, 23.10.2020 01:01

Social Studies, 23.10.2020 01:01

Social Studies, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01

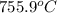 this reaction is spontaneous under standard conditions.
this reaction is spontaneous under standard conditions.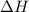 = 131.3 KJ/mole = 131300 J/mole
= 131.3 KJ/mole = 131300 J/mole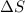 = 127.6 J/mole.K
= 127.6 J/mole.K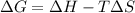
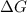 is negative or we can say that the value of
is negative or we can say that the value of