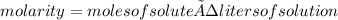Chemistry, 09.12.2019 18:31 jonmorton159
Urea, (nh2)2co, is a product of metabolism of proteins. an aqueous solution is 37.2% urea by mass and has a density of 1.032 g/ml. calculate the molarity of urea in this solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3


Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
Urea, (nh2)2co, is a product of metabolism of proteins. an aqueous solution is 37.2% urea by mass an...
Questions

History, 13.11.2020 06:20

Mathematics, 13.11.2020 06:20


Mathematics, 13.11.2020 06:20

History, 13.11.2020 06:20


History, 13.11.2020 06:20

English, 13.11.2020 06:20

History, 13.11.2020 06:20

Mathematics, 13.11.2020 06:20

Biology, 13.11.2020 06:20

Mathematics, 13.11.2020 06:20


History, 13.11.2020 06:20

History, 13.11.2020 06:20

Health, 13.11.2020 06:20

Mathematics, 13.11.2020 06:20


Mathematics, 13.11.2020 06:20

English, 13.11.2020 06:20