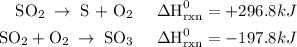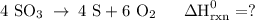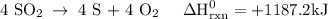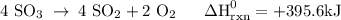Chemistry, 09.12.2019 18:31 ahhhhhhhh5509
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: 4 so3(g) → 4 s(s) + 6 o2(g) δh°rxn = ? given: so2(g) → s(s) + o2(g) δh°rxn = +296.8 kj 2 so2(g) + o2(g) → 2 so3(g) δh°rxn = -197.8 kj

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

You know the right answer?
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: 4...
Questions


English, 04.12.2020 23:10


Mathematics, 04.12.2020 23:10

Mathematics, 04.12.2020 23:10



Health, 04.12.2020 23:10

Mathematics, 04.12.2020 23:10

Spanish, 04.12.2020 23:10

Mathematics, 04.12.2020 23:10

Mathematics, 04.12.2020 23:10

Mathematics, 04.12.2020 23:10


Biology, 04.12.2020 23:10

Mathematics, 04.12.2020 23:10



Mathematics, 04.12.2020 23:10

History, 04.12.2020 23:10

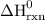 = 1582.8 kJ.
= 1582.8 kJ.