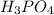Chemistry, 09.12.2019 19:31 gloverj9639
Phosphoric acid, which is a component of many soft drinks, has a molar mass of 98.00 grams per mole. a sample of phosphoric acid contains 3.1 percent hydrogen, 31.6 percent phosphorus, and 65.3 percent oxygen by mass. what is the molecular formula of phosphoric acid

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Phosphoric acid, which is a component of many soft drinks, has a molar mass of 98.00 grams per mole....
Questions



Social Studies, 27.08.2019 18:30


Social Studies, 27.08.2019 18:30




Social Studies, 27.08.2019 18:30

Physics, 27.08.2019 18:30

English, 27.08.2019 18:30

English, 27.08.2019 18:30

Mathematics, 27.08.2019 18:30




Mathematics, 27.08.2019 18:30

Mathematics, 27.08.2019 18:30


Computers and Technology, 27.08.2019 18:30