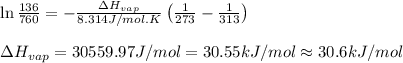Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
The vapor pressure of dichloromethane, ch2cl2, at 0 ∘c is 134 mmhg. the normal boiling point of dich...
Questions



Biology, 29.09.2019 20:10

Mathematics, 29.09.2019 20:10


Mathematics, 29.09.2019 20:10


Mathematics, 29.09.2019 20:10

History, 29.09.2019 20:10


Mathematics, 29.09.2019 20:10

Biology, 29.09.2019 20:10



Mathematics, 29.09.2019 20:10

Biology, 29.09.2019 20:10

English, 29.09.2019 20:10

Chemistry, 29.09.2019 20:10

Mathematics, 29.09.2019 20:10

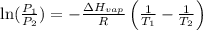
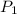 = vapor pressure at temperature
= vapor pressure at temperature 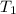 = 134 mmHg
= 134 mmHg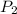 = vapor pressure at temperature
= vapor pressure at temperature 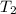 (atmospheric pressure) = 760 mmHg
(atmospheric pressure) = 760 mmHg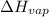 = molar heat of vaporization
= molar heat of vaporization![0^oC=[273+0]K=273K](/tpl/images/0410/2746/614a3.png)
![40^oC=[273+40]K=313K](/tpl/images/0410/2746/c9054.png)