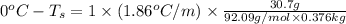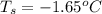Chemistry, 09.12.2019 19:31 nayelimoormann
Determine the freezing point depression of a solution that contains 30.7 g glycerin (c3h8o3, molar mass = 92.09 g/mol) in 376 ml of water. some possibly useful constants for water are kf = 1.86°c/m and kb = 0.512°c/m.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
Determine the freezing point depression of a solution that contains 30.7 g glycerin (c3h8o3, molar m...
Questions


Mathematics, 24.01.2021 07:20



Mathematics, 24.01.2021 07:20

Computers and Technology, 24.01.2021 07:20

History, 24.01.2021 07:20




Mathematics, 24.01.2021 07:20

Chemistry, 24.01.2021 07:20

Mathematics, 24.01.2021 07:20

English, 24.01.2021 07:20

Mathematics, 24.01.2021 07:20

Computers and Technology, 24.01.2021 07:20

History, 24.01.2021 07:20

Arts, 24.01.2021 07:20

Arts, 24.01.2021 07:20

Arts, 24.01.2021 07:20

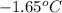
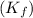 for water =
for water = 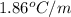

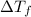 = change in freezing point
= change in freezing point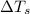 = freezing point of solution = ?
= freezing point of solution = ?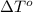 = freezing point of water =
= freezing point of water = 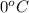
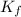 = freezing point constant for water =
= freezing point constant for water =