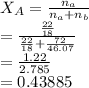Chemistry, 09.12.2019 19:31 historyfanboy101
The vapor pressure of pure water at 15 °c is 12.8 mm hg. what is the equilibrium vapor pressure of water above a mixture of 72.0 g ethanol (ch3ch2oh, molar mass = 46.07 g/mol) and 22.0 g water?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
The vapor pressure of pure water at 15 °c is 12.8 mm hg. what is the equilibrium vapor pressure of w...
Questions


Mathematics, 07.10.2019 21:30

English, 07.10.2019 21:30

Mathematics, 07.10.2019 21:30




Mathematics, 07.10.2019 21:30




English, 07.10.2019 21:30





Mathematics, 07.10.2019 21:30



Biology, 07.10.2019 21:30

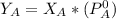
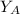 is the partial vapour pressure ( mm Hg)
is the partial vapour pressure ( mm Hg)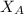 is the mole fraction
is the mole fraction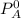 is the pure vapour pressure = 12.8 mm Hg
is the pure vapour pressure = 12.8 mm Hg