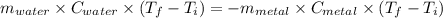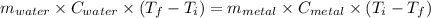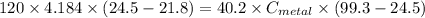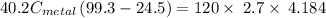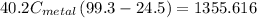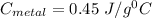Chemistry, 09.12.2019 20:31 minosmora01
A40.2 g sample of a metal heated to 99.3â°c is placed into a calorimeter containing 120 g of water at 21.8â°c. the final temperature of the water is 24.5â°c. which of the following might be the metal that was used?
a) aluminum (c=0.89j/gâ°c)
b) iron (c=0.45j/gâ°c)
c) copper (0.20j/gâ°c)
d) lead (c=0.14j/gâ°c)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Consider the balanced equation below. n2h4 + 2h2o2 n2 + 4h2o what are the mole ratios of hydrazine (n2h4) to hydrogen peroxide (h2o2) and hydrazine to water? 1: 2 and 1: 4 1: 3 and 1: 4 1: 2 and 3: 5 1: 3 and 3: 5
Answers: 3

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
A40.2 g sample of a metal heated to 99.3â°c is placed into a calorimeter containing 120 g of water a...
Questions

Mathematics, 25.09.2019 19:00

English, 25.09.2019 19:00

English, 25.09.2019 19:00












English, 25.09.2019 19:00

English, 25.09.2019 19:00




English, 25.09.2019 19:00