
Chemistry, 09.12.2019 20:31 saabrrinnaaa
Calculate the work, w, gained or lost by the system when a gas expands from 15 l to 45 l against a constant external pressure of 1.5 atm. 1 l × atm = 101 j.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
Calculate the work, w, gained or lost by the system when a gas expands from 15 l to 45 l against a c...
Questions








Mathematics, 09.04.2021 20:20

Mathematics, 09.04.2021 20:20

Mathematics, 09.04.2021 20:20



Mathematics, 09.04.2021 20:20


Mathematics, 09.04.2021 20:20

Mathematics, 09.04.2021 20:20

Mathematics, 09.04.2021 20:20

Mathematics, 09.04.2021 20:20

Mathematics, 09.04.2021 20:20

Mathematics, 09.04.2021 20:20

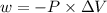
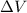 is the change in volume
is the change in volume
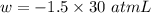
 (negative sign implies work is done by the system)
(negative sign implies work is done by the system)

