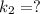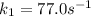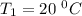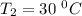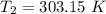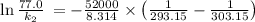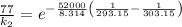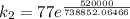Chemistry, 09.12.2019 20:31 brenda0113hernandez
The rate constant for a certain chemical reaction is 77.0 s-1 at a temperature of 20.0°c. if the activation energy for this reaction is 52. kj/mole, what is the rate constant at a temperature of 30.0°c?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
You know the right answer?
The rate constant for a certain chemical reaction is 77.0 s-1 at a temperature of 20.0°c. if the act...
Questions





Mathematics, 08.12.2019 06:31


Mathematics, 08.12.2019 06:31


Mathematics, 08.12.2019 06:31


Mathematics, 08.12.2019 06:31







Mathematics, 08.12.2019 06:31

Mathematics, 08.12.2019 06:31

Mathematics, 08.12.2019 06:31

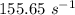 is the rate constant at a temperature of 30.0 °C.
is the rate constant at a temperature of 30.0 °C.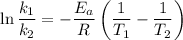
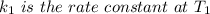
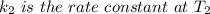
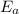 is the activation energy
is the activation energy