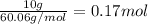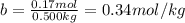Chemistry, 09.12.2019 20:31 alex12everett
The molal freezing point depression constant =kf6.19·°c·kgmol−1 for a certain substance x . when 10.g of urea nh22co are dissolved in 500.g of x , the solution freezes at −6.8°c . calculate the freezing point of pure x . be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
The molal freezing point depression constant =kf6.19·°c·kgmol−1 for a certain substance x . when 10....
Questions

English, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

History, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Physics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

English, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01