

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2


Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Sodium carbonate can be made by heating sodium bicarbonate: 2nahco3(s) → na2co3(s) + co2(g) + h2o(g...
Questions

Arts, 07.04.2020 02:54






Physics, 07.04.2020 02:54

Chemistry, 07.04.2020 02:55



English, 07.04.2020 02:55





Mathematics, 07.04.2020 02:55

Mathematics, 07.04.2020 02:55

Physics, 07.04.2020 02:55


English, 07.04.2020 02:55

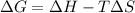
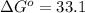
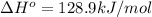
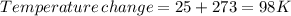
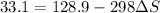
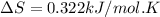
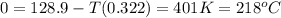
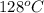 minimum temperature will the reaction become spontaneous.
minimum temperature will the reaction become spontaneous.

