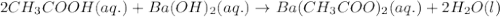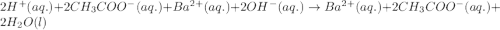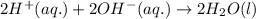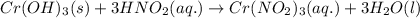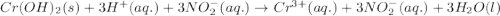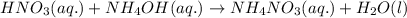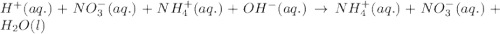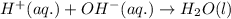Chemistry, 23.11.2019 09:31 sepdentalcare8774
Write the balanced molecular and net ionic equations for each of the following neutralization reactions. (a) aqueous acetic acid (hc2h3o2) is neutralized by aqueous barium hydroxide. (b) solid chromium(iii) hydroxide reacts with nitrous acid. (c) aqueous nitric acid and aqueous ammonia react.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Write the balanced molecular and net ionic equations for each of the following neutralization reacti...
Questions

English, 18.11.2019 03:31


History, 18.11.2019 03:31


Mathematics, 18.11.2019 03:31

Mathematics, 18.11.2019 03:31




Mathematics, 18.11.2019 03:31







Mathematics, 18.11.2019 03:31


Medicine, 18.11.2019 03:31

Mathematics, 18.11.2019 03:31

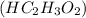 is neutralized by aqueous barium hydroxide.
is neutralized by aqueous barium hydroxide.