
Chemistry, 09.12.2019 21:31 trodrickwilliams2019
Match each transition metal ion with its condensed ground-state electron configuration. a [ar]3d2 b [ar]4s23d3 c [kr]4d10 d [xe] e [ar]3d6 f [ar]3d5 g [xe]4f145d10 mn2+ hg2+ la3+ fe3+ ag+ co3+

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1


Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
Match each transition metal ion with its condensed ground-state electron configuration. a [ar]3d2 b...
Questions

Mathematics, 24.09.2019 03:30

History, 24.09.2019 03:30



Mathematics, 24.09.2019 03:30

Social Studies, 24.09.2019 03:30




Mathematics, 24.09.2019 03:30

Mathematics, 24.09.2019 03:30




English, 24.09.2019 03:30





Mathematics, 24.09.2019 03:30

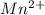 : - F .
: - F . ![[Ar]3d^{5}](/tpl/images/0410/5116/2ab95.png)
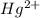 : - G.
: - G. ![[Xe]4f^{14}5d^{10}](/tpl/images/0410/5116/8605c.png)
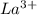 : - D.
: - D. ![[Xe]](/tpl/images/0410/5116/8b32c.png)
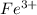 : - F.
: - F. 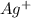 : - C.
: - C. ![[Kr]4d^{10}](/tpl/images/0410/5116/f3cc7.png)
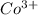 : - E.
: - E. ![[Ar]3d^{6}](/tpl/images/0410/5116/214ec.png)
![[Ar]3d^{5}4s^2](/tpl/images/0410/5116/9fae5.png)
![[Xe]4f^{14}5d^{10}6s^2](/tpl/images/0410/5116/13e4d.png)
![[Xe]5d^{1}6s^2](/tpl/images/0410/5116/43998.png)
![[Ar]3d^{6}4s^2](/tpl/images/0410/5116/067fb.png)
![[Kr]4d^{10}5s^1](/tpl/images/0410/5116/5679a.png)
![[Ar]3d^{7}4s^2](/tpl/images/0410/5116/dc67c.png)



