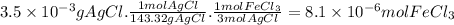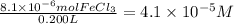Chemistry, 09.12.2019 21:31 tripleog12716
One way the u. s. environmental protection agency (epa) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(iii) chloride, which would react with silver nitrate solution like this: fecl3(aq) + 3agno3(aq) → 3agcl(s) + feno33(aq) the chemist adds 57.0mm silver nitrate solution to the sample until silver chloride stops forming. she then washes, dries, and weighs the precipitate. she finds she has collected 3.5mg of silver chloride. calculate the concentration of iron(iii) chloride contaminant in the original groundwater sample. round your answer to 2 significant digits.

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 1
You know the right answer?
One way the u. s. environmental protection agency (epa) tests for chloride contaminants in water is...
Questions



Mathematics, 02.04.2020 21:15

Biology, 02.04.2020 21:15



Mathematics, 02.04.2020 21:15


Spanish, 02.04.2020 21:15

Chemistry, 02.04.2020 21:15


History, 02.04.2020 21:16






Mathematics, 02.04.2020 21:16

Mathematics, 02.04.2020 21:16