
Chemistry, 09.12.2019 21:31 Knownothing
Fe(ii) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the solution, which converts fe(ii) to insoluble fe(iii):
4fe(oh)+(aq)+ 4oh-(aq)+o2(g)+2h2o(l) = 4fe(oh)3(s)
how many grams of o2 are consumed to precipitate all of the iron in 85ml of 0.075 m fe(ii).
explain step by step

Answers: 3
Another question on Chemistry

Chemistry, 20.06.2019 18:02
Heat by radiation will most likely be transferred through a.gas b.liquid c.solid d.insulator
Answers: 1

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2
You know the right answer?
Fe(ii) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the sol...
Questions







Mathematics, 03.03.2020 21:30




English, 03.03.2020 21:30



Biology, 03.03.2020 21:30

Mathematics, 03.03.2020 21:30





Mathematics, 03.03.2020 21:30

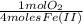 =
= 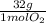 = 0,051g of O₂
= 0,051g of O₂

