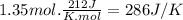Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1
You know the right answer?
The normal boiling point of methanol is 64.7 ∘ c and the molar enthalpy of vaporization is 71.8kj/mo...
Questions




Mathematics, 12.02.2021 17:30

English, 12.02.2021 17:30

History, 12.02.2021 17:30

Advanced Placement (AP), 12.02.2021 17:30


Mathematics, 12.02.2021 17:30

Mathematics, 12.02.2021 17:30

History, 12.02.2021 17:30

English, 12.02.2021 17:30


Mathematics, 12.02.2021 17:30



Mathematics, 12.02.2021 17:30

Mathematics, 12.02.2021 17:30